近日,昆明理工大学材料科学与工程学院陈江照教授团队在国际化学顶级期刊Angewandte Chemie-International Edition(IF=16.1)上以题为“Simultaneous suppression of multilayer ion migration via molecular complexation strategy toward high-performance regular perovskite solar cells”发表最新研究成果,该研究工作得到国家自然科学基金面上、兵团重点领域科技攻关计划等项目的资助。
在环境与能源领域,全球气候变化的加剧促使各国决策者将可再生能源的开发和应用视为重中之重。许多国家相继出台减少碳排放的政策,为太阳能技术的进步创造了良好的发展环境。太阳能作为高效清洁能源,在实现这些目标中发挥着关键作用,成为推动可持续发展的重要因素。尤其是钙钛矿太阳能电池(PSC),由于其独特的光电特性、低成本的制造工艺和多功能的应用场景,在过去十年中引起了研究人员的广泛关注。虽然PSC在实验室的功率转换效率(PCE)已经超过26%,但在实际应用中仍面临许多限制和挑战。一方面,目前最先进的PCE仍未达到理论上的Shockley-Queisser极限。需要探索有效的策略来最大限度地减少非辐射复合损失,以进一步克服效率提升的瓶颈。另一方面,较差的长期稳定性对钙钛矿光伏技术的商业化应用提出了严峻的挑战。
目前,n-i-p正式PSC的稳定性被广泛证明不如p-i-n反式PSC,这主要是由于高效n-i-p器件中常常使用掺有双(三氟甲烷)磺酰亚胺锂盐(Li-TFSI)和4-叔丁基吡啶(tBP)的2,2',7,7'-四[N,N-二(4-甲氧基苯基)氨基]-9,9'-螺二芴(Spiro-OMeTAD)空穴传输层(HTL)。这些掺杂剂吸湿性极强,导致HTL和最终器件的防潮能力很差。此外,锂离子(Li+)在整个器件内的迁移和扩散会降低其光和热稳定性。为了解决Li+扩散引起的不稳定性,研究者深入研究了各种类型的策略,主要包括无掺杂空穴传输材料(HTM)(例如PM6、TAT-2T-CAN、PC3、和P3HT)和Li-TFSI的替代掺杂剂(例如TBMP+TFSI-和Zn-TFSI2)。尽管如此,使用无掺杂剂HTM或替代Li-TFSI掺杂剂的器件的PCE仍然低于Li-TFSI掺杂的器件。因此,开发新的高效共掺杂剂以抑制Li+扩散来稳定Spiro-OMeTAD HTL势在必行。众所周知,碘离子(I-)会从钙钛矿层扩散到HTL和银电极之间的界面,金属电极中的Ag原子也会通过HTL扩散到钙钛矿层。Ag和I-的交叉扩散是PSC退化的主要原因之一。简而言之,Li+、I-和Ag在不同功能层中的迁移和扩散极大地阻碍了PSC的进一步发展。除了稳定性之外,界面陷阱辅助非辐射复合和HTL相对较低的电导率也会降低PSC的PCE。
迄今为止,人们已经为解决这些关键挑战做出了巨大努力,并取得了实质性进展。例如,Wang等人在钙钛矿/Spiro-OMeTAD界面处引入了4-甲磺酰基苯甲脒盐酸盐(MSBH),并证明MSBH中的磺酰基与Li+的强配位作用可有效抑制Li+离子的迁移。此外,Li等人在Spiro-OMeTAD HTL中引入了12-Crown-4,并证明12-Crown-4 可以通过主客体相互作用抑制Li⁺迁移,从而显著提高水分和热稳定性。Yang等人用氧化钼(HxMoO₃)修饰HTL/Ag界面以抑制Ag扩散。我们团队开发了一种基于多齿配体绿色生物材料2-脱氧-2,2-二氟-d-赤式-五呋喃-1-酮-3,5-二苯甲酸酯(DDPUD)的分子锁策略,通过抑制I⁻迁移和钝化配位不足的Pb²⁺缺陷来稳定钙钛矿/HTL界面。然而,上述方法仅抑制了Li+、Ag或I-的迁移。最近,为了增强修饰分子的功能,我们团队利用L-谷氨酸二苄酯4-甲苯磺酸盐(GADET)来稳定HTL和钙钛矿/HTL界面。我们证明了对甲苯磺酸盐与Li+的配位抑制了Li+的迁移,而分子中的-NH3+可以与I-形成氢键,从而抑制I-的迁移。尽管如此,考虑到这些迁移物种来自三个不同的功能层,用一种简单有效的方法同时抑制Li+、I-和Ag的迁移仍然具有挑战性。直接将共掺杂剂掺入HTL可能提供一种可行有效的解决方案,以同时解决上述挑战。配位不足的Pb2+和/或卤化物空位缺陷可以为碘离子的迁移提供途径。可以通过钝化配位不足的Pb2+和/或卤化物空位缺陷来抑制碘迁移。研究人员已经采用了主客体络合策略来钝化配位不足的Pb2+缺陷、抑制Li+迁移、调节钙钛矿结晶或稳定钙钛矿前驱体溶液。但目前尚无关于通过主客体络合同时抑制Li⁺、I⁻和Ag迁移的报道,这表明主客体络合策略的功能仍需进一步扩展,以充分发挥其在多层离子迁移调控中的潜力。鉴于Ag、Li⁺和配位不足的Pb2+等活性移动化学物质均为缺电子的Lewis酸,开发一种具有强配位能力的Lewis碱配体与这些移动离子进行有效配位,有望实现对多层多种移动化学物质的同时抑制,从而提高PSCs的长期稳定性。因此,利用Lewis碱配体通过主客体络合作用同时抑制Li+、I-和Ag的迁移应该是可行的。
鉴于此,陈江照教授团队提出了一种多功能主客体络合策略,将路易斯碱配体双(2,4,6-三氯苯基)草酸酯(TCPO)掺入Spiro-OMeTAD HTL,通过主客体络合相互作用,TCPO可以同时抑制Li+、I-和Ag的迁移和扩散,从而稳定钙钛矿层、HTL和银电极及其界面。此外,TCPO促进了Spiro-OMeTAD的掺杂过程,加速了空穴提取和转移。TCPO掺杂后,钙钛矿薄膜表面缺陷减少,陷阱辅助的非辐射复合降低。引入两个疏水三氯苯基增强了HTL的防潮性,并保护了底层钙钛矿薄膜免受环境空气中水分的侵袭。掺杂TCPO的正式器件实现了25.68%的冠军PCE(认证效率为 25.59%)。未封装的TCPO掺杂器件在最大功率点(MPP)连续运行730小时后衰减仅9.98%,在30%相对湿度(RH)的环境空气中老化2800小时后仍能保持初始效率的94.1%,这是迄今为止正式PSC报道的最高稳定性之一。该研究显著提高了正式器件的稳定性,为后续相关研究和商业应用奠定了坚实的基础。该工作通过合理设计多功能Lewis碱配体分子,为通过主客体络合策略提高PSC的光伏性能和长期稳定性提供了有价值的参考和指导。
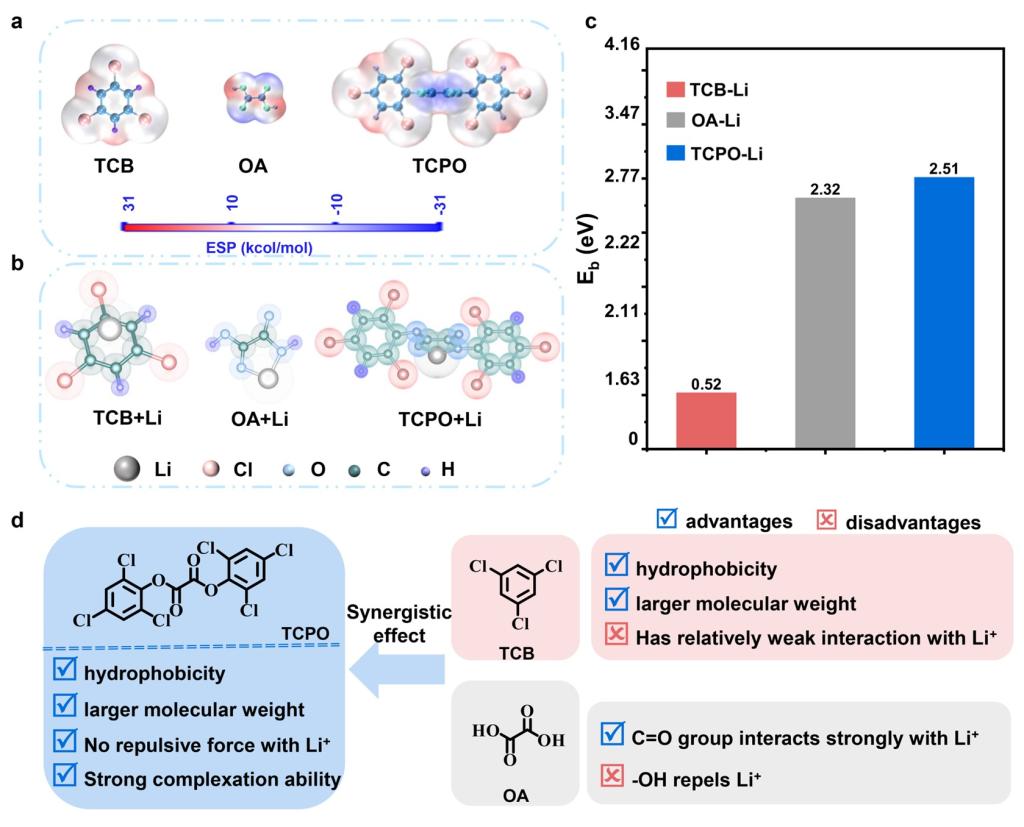
Figure 1. Theoretical insights into inhibition mechanisms of mobile Li+ ions. (a) ESP images of TCB, OA, and TCPO. Molecular structures (b) and binding energies (c) of Li+ with TCB, OA, and TCPO calculated and optimized using density functional theory. (d) Design thought of TCPO ligand molecules used for host-guest complexation interaction.
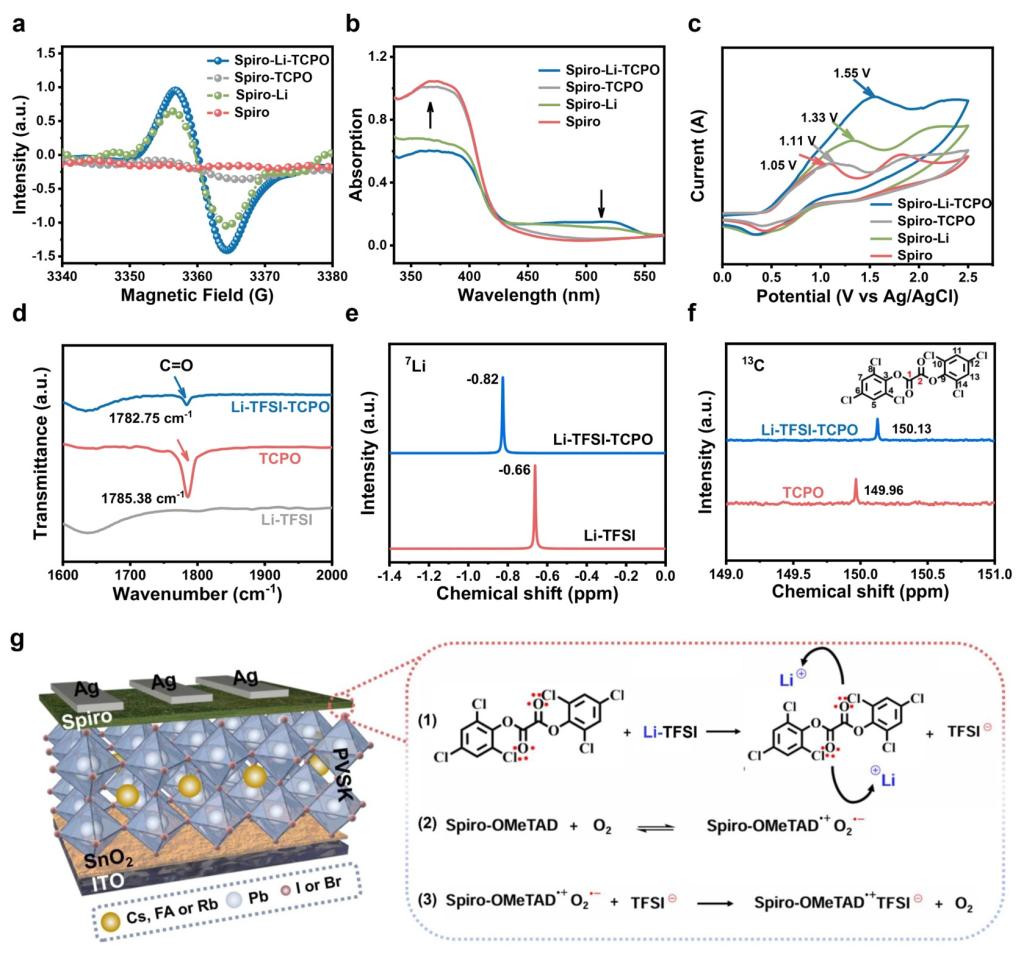
Figure 2. Promoted p-doping by TCPO and chemical interaction of TCPO with Li+ ion. (a) ESR spectra of Spiro, Spiro-TCPO, Spiro-Li, and Spiro-Li-TCPO solutions. (b) UV-visible absorption spectra of Spiro, Spiro-TCPO, Spiro-Li and Spiro-Li-TCPO films. (c) Cyclic voltammograms of Spiro, Spiro-TCPO, Spiro-Li, and Spiro-Li-TCPO. (d) FTIR spectra of Li-TFSI, TCPO and TCPO-Li-TFSI in the wavenumber range of 1600-2000 cm-1. (e) 7Li NMR of Li-TFSI solutions with and without TCPO. (f) 13C NMR of TCPO solutions with and without Li-TFSI. (g) Schematic illustration of the mechanisms of p-doping promotion and Li+ migration suppression via TCPO.
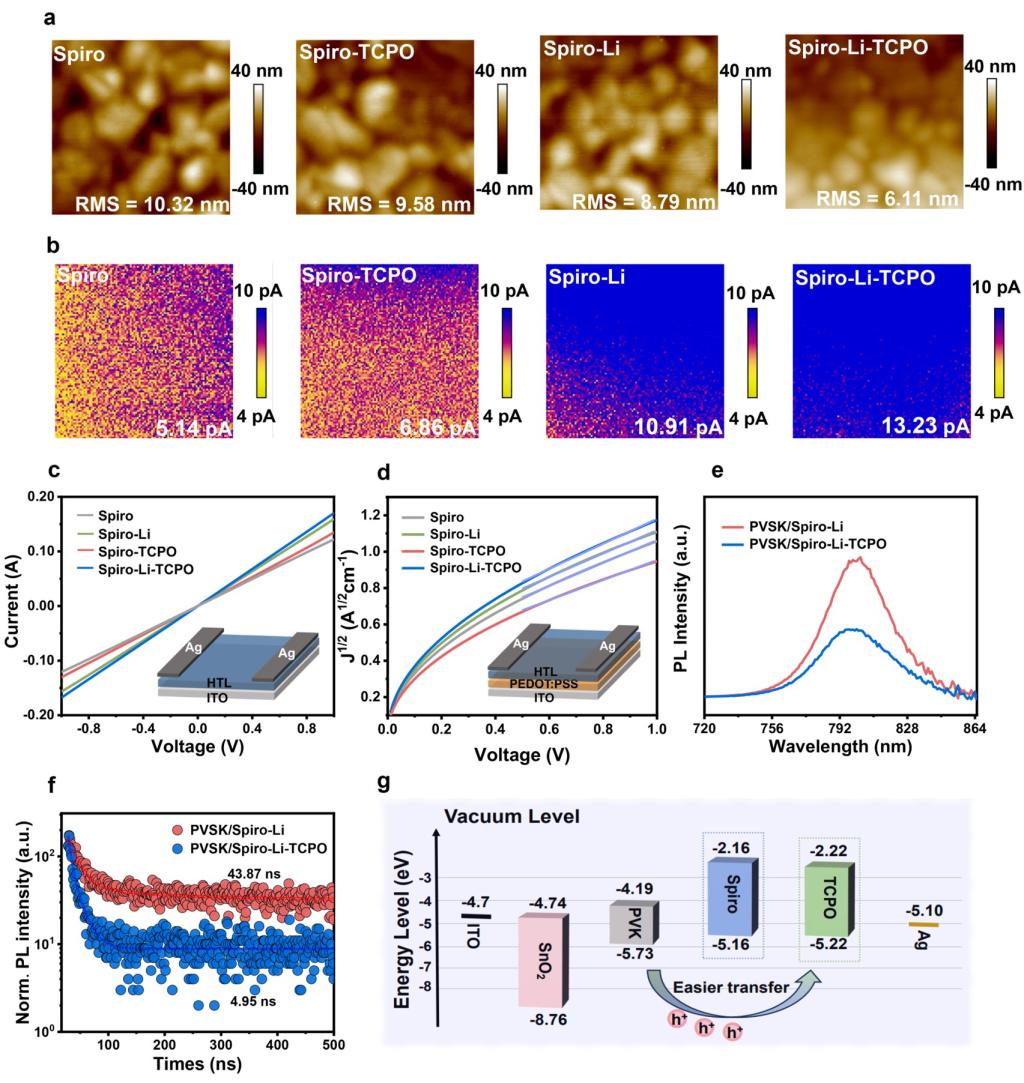
Figure 3. Characterization of HTLs without and with TCPO. AFM (a) and c-AFM (b) images of pure Spiro, Spiro-TCPO, Spiro-Li, and Spiro-Li-TCPO. The size of the images is 2×2 μm2. Conductivities (c) and hole mobilities (d) of pure Spiro, Spiro-TCPO, Spiro-Li, and Spiro-Li-TCPO. PL (e) and TRPL (f) spectra of perovskite films coated with Spiro-Li and Spiro-Li-TCPO films on glass substrates. PVSK stands for perovskite. (g) Energy level arrangement of Spiro-Li films with and without TCPO.

Figure 4. Simultaneous suppression of Li+, I- and Ag migration via host-guest complexation. (a) Photographs of the perovskite films with Spiro-Li (control) and Spiro-Li-TCPO (target) under damp and heat environment (temperature 85 ℃, relative humidity 61%) after aging for 1, 2, 4, 6 and 8 days. (b) Water contact angles of the perovskite films with Spiro-Li and Spiro-Li-TCPO. (c) Cross-sectional SEM images of the perovskite films with Spiro-Li and Spiro-Li-TCPO under damp and heat environment (temperature 85 ℃, relative humidity 61%) before and after aging for 8 days. (d) TOF-SIMS for the control and target devices after exposing to one sun and heating at 65 °C for 8 days. (e) TOF-SIMS 3D reconstruction of CsAg+, Cs2I-and Li+ ions. (f) Schematic diagram of suppressing I-, Li+, and Ag migration through TCPO.
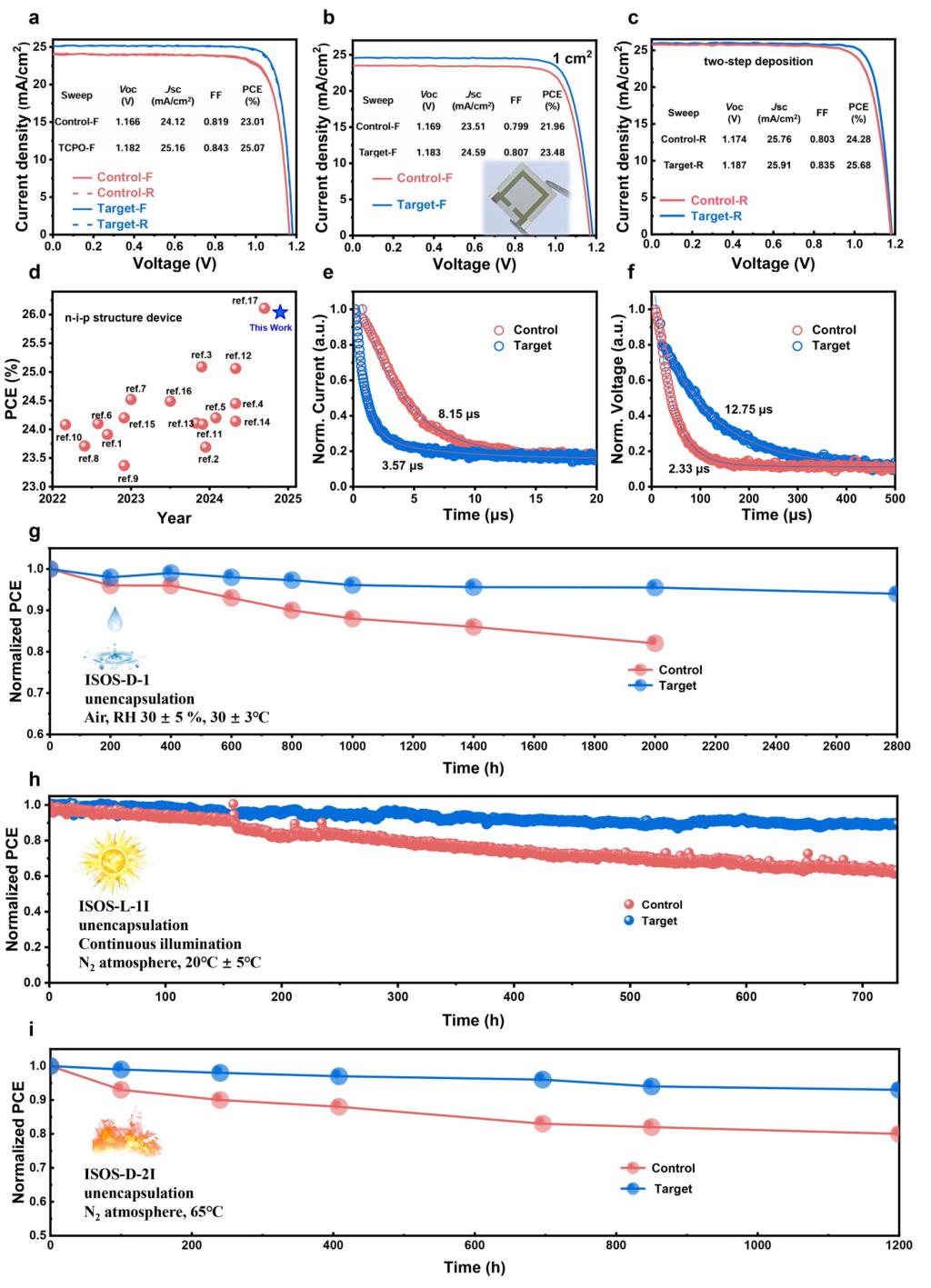
Figure 5. Photovoltaic performance and long-term stability of devices without and with TCPO. (a) J-V curves of the best-performing devices without and with TCPO. (b) J-V curves of the champion control and target devices with an active area of 1 cm2. (c) J-V curves of the best-performing devices without and with TCPO prepared using a two-step perovskite deposition approach (active area 0.08 cm2). (d) Comparison of the PCEs for our device and reported highly efficient n-i-p structure PSCs. TPC (e) and TPV (f) curves for the control and target devices. (g) Humidity stability of the control and modified PSCs in a light-shielded environment at 25-35% relative humidity. (h) Operation stability of the unencapsulated control and target devices at MPP under continuous 1 sun irradiation. (i) Thermal stability of the unencapsulated control and modified devices heated at 65 °C in a dark nitrogen glove box.
Qian Zhou#, Yingying Yang#, Dongmei He*, Ke Yang, Yue Yu, Xinxing Liu, Jiajia Zhang*, Xuxia Shai, Jinsong Wang, Jianhong Yi, Meicheng Li*, Jiangzhao Chen*. Simultaneous suppression of multilayer ion migration via molecular complexation strategy toward high-performance regular perovskite solar cells. Angew. Chem. Int. Ed. 2024, e202416605.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202416605

